 Radioactive decay
Radioactive decay
The nucleus of an atom is described by its proton number (Z, characteristic of the chemical species of the element), and its nucleon number (A or mass number). An atom of the same chemical species may contain a different number of nucleons, ie a different number of neutrons (N = A - Z). Isotopes are atoms which have the same number of protons but not the same number of neutrons. Isotopes of an atom all have the same chemical properties (same Z number) and remain in constant proportion in nature.
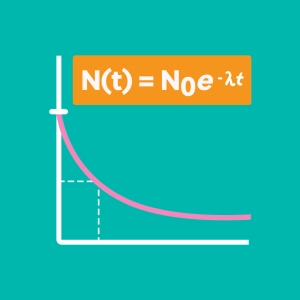
A nucleus is stable when the nuclear force compensates the electrical repulsion force between the positively charged protons. Some nuclei are unstable. They naturally release energy (radiation) generating new nuclei that can be stable or unstable. This decay is random, and it depends on the nature of the element: this is radioactivity.
The half-life of a given isotope is the amount of time it takes for half of the atoms in a sample to decay. This simulation allows you to address, using three different isotopes, notions like radioactive decay, carbon dating, half life constant.
